3D Printed Micronized Fat-Laden Collagen Constructs for Treatment of Chronic Wounds
Trevor Schmitt 1 , Nathan Katz 2 , Vipuil Kishore 1 .
1 Department of Biomedical and Chemical Engineering and Sciences, Florida Institute of Technology, Melbourne, FL 32901
2 Jointechlabs Inc., N. Barrington, IL 60010
Statement of Purpose: Chronic skin wounds such as diabetic ulcers and pressure ulcers have high prevalence in patients with poor circulation, neuropathy, and infection. 1 A regenerative approach using a combination of adipose stem cells (ASCs) and fat-derived stromal vascular fraction (SVF) can be an effective strategy for healing of chronic wounds via regulation of growth factors and cytokines, targeted cell response, and new blood vessel formation. 2,3 In this realm, we have developed a breakthrough device (Mini-Stem System, Jointechlabs) capable of processing patient-derived lipoaspirates to produce micronized fat graft and purified SVF in a single closed loop system (Fig. 1A). The micronized fat graft consists of a rich fraction of ASCs and endothelial cell progenitors preserved in their native niche without excess oil and debris. To the best of our knowledge, the current study is the first attempt to combine micronized fat with a polymeric ink to 3D print fat-laden crosslinkable constructs with high print fidelity and stability. 3D bioprinting will allow for encapsulation of precise ratios of micronized fat into anatomically complex structures that can be applied to heterogeneous wound sites of varying depth and complexity for complete and expedited healing of chronic wounds.
Methods: Lipoaspirate collected from donors was processed using the Mini-Stem system to obtain the micronized fat and cryopreserved in liquid nitrogen until use. Cell count and viability was assessed by digesting the
micronized fat with collagenase and analyzing the harvested cells using a Muse Cell Analyzer. For bioink formulation, cryopreserved micronized fat was warmed to 37 ºC in a water bath and mixed at different concentrations
(i.e., 10%, 30%, 50% w/w) with 6 mg/mL methacrylated collagen solution (Advanced Biomatrix) containing 1% w/v water soluble azo photoinitiator (VA-086). 3D bioprinting of micronized fat-laden collagen constructs was performed using an extrusion-based Regemat 3D bioprinter and a custom 3D model (Fig. 1B). Briefly, methacrylated collagen bioink with different concentrations of micronized fat was extruded into a freeform reversible embedding of suspended hydrogels (FRESH) support bath in a sterile manner and photochemically crosslinked via UV exposure for 1 min (365nm, 17 mW/cm 2 ). 4 Following this, the printed construct was incubated at 37 ºC to melt the support bath and recover the construct. Micronized fat-laden constructs were cultured in growth medium (DMEM+10%FBS) for 15 days. Alamar blue assay was performed to assess cell viability and metabolic activity. At periodic intervals, culture medium was collected and analyzed by ELISA to quantify the cell-secreted soluble factors.
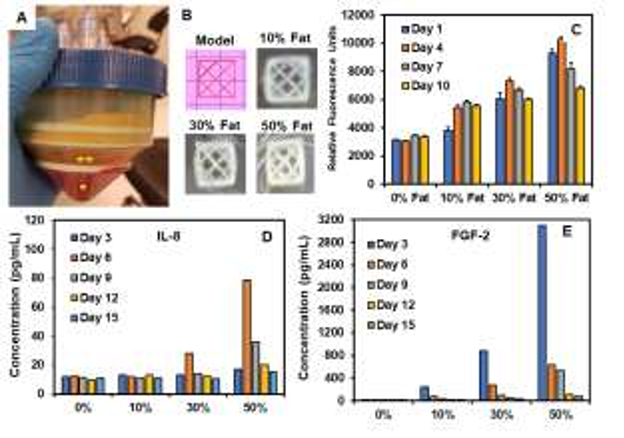
Results: Flow cytometry results showed a cell count ranging from 1 x10 6 to 5×10 6 cells/ml of micronized fat with a viability of > 90% (data not shown). Micronized fatladen 3D bioprinted constructs matched the 3D model and retained their shape fidelity for the entire culture duration (Fig. 1B). Alamar blue showed that cell metabolic activity correlated with the amount of fat content at day 1 and was observed to increase on constructs with 30% and 50% micronized fat on day 4 followed by a decrease for the rest of the culture (Fig. 1C). For constructs with 10% Fat, cell metabolic activity was found to increase until day 7. Results from ELISA showed temporal changes in the expression of key cytokines known to play a critical role in wound healing process. Specifically, expression of interleukin-8 (IL-8), a key cytokine for neutrophil recruitment, was found to peak at day 6 for constructs with high micronized fat concentration (Fig. 1D). On the other hand, expression of fibroblast growth factor (FGF-2) showed a fat concentration dependent peak at day 3 followed by a decrease for the culture duration (Fig. 1E).
Figure 1: (A) Mini-Stem System for separation of lipoaspirate components (* – red blood cells and debris, ** – micronized fat fraction). (B) 3D printed collagen meshes with different concentrations of micronized fat. (C) Alamar blue assay for cell metabolic activity over time. (D, E) ELISA for quantification of soluble factors – IL-8 and FGF-2 in culture medium.
Conclusions: The results of this feasibility study demonstrate that 3D printed micronized-fat laden collagen constructs hold promise for use in wound healing applications. Since the cell viability is limited up to one week, reapplication of the micronized fat graft on a weekly basis would be an effective strategy to achieve continued and efficient healing of chronic wounds. This is a highly feasible approach since micronized fat from patient-
derived adipose tissue can be cryopreserved without loss in cell viability. Future studies will assess the neovascularization potential of 3D printed micronized fat-laden constructs in vitro and in vivo using a mouse model.
References:
1 Gefen, Medical Engineering and Physics, 2019 ;
2 Tabit et al., Aesth Plast Surg, 2012 ;
3 Deng et al.,Medicine, 2018.
4 Kajave et al., Biomedical Materials, 2020.